Camber Pharmaceuticals Launches Generic Alesse®
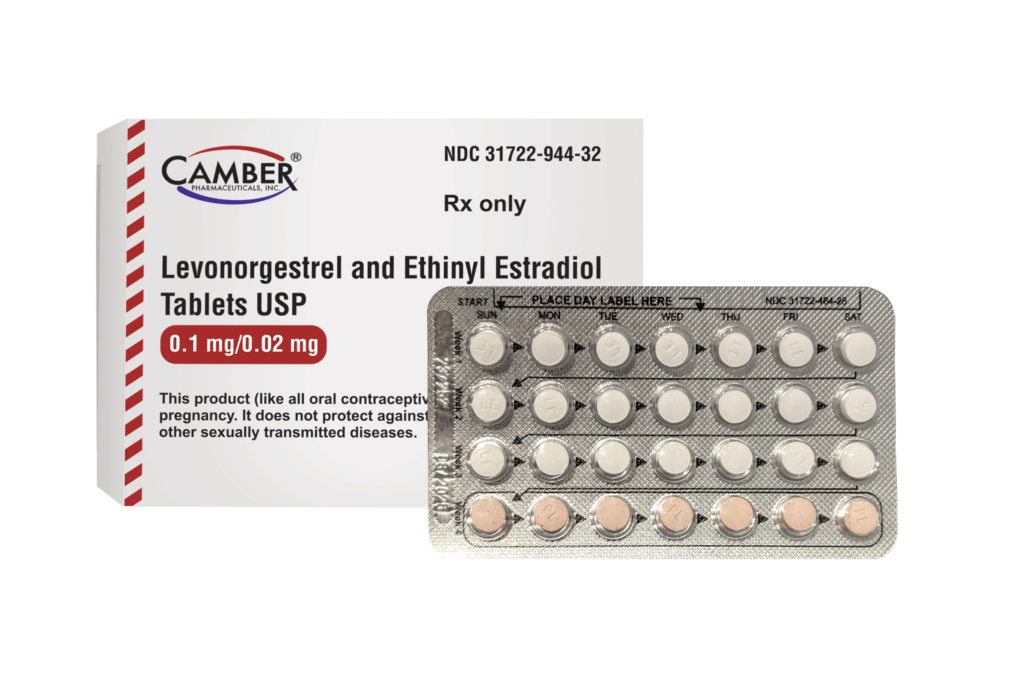
Piscataway, NJ, April 4, 2024–Camber Pharmaceuticals is pleased to announce the addition of Levonorgestrel and Ethinyl Estradiol Tablets USP to its current portfolio.
Levonorgestrel and Ethinyl Estradiol Tablets USP are indicated for the prevention of pregnancy in women who elect to use oral contraceptives.
Levonorgestrel and Ethinyl Estradiol Tablets USP (0.1 mg/0.02 mg) is available in boxes of 3 blister packs of 28 tablets/packet.
To find out more about Levonorgestrel and Ethinyl Estradiol Tablets USP, please visit https://www.camberpharma.com/levonorgestrel-ee