Camber Launches Eslicarbazepine Acetate Tablets
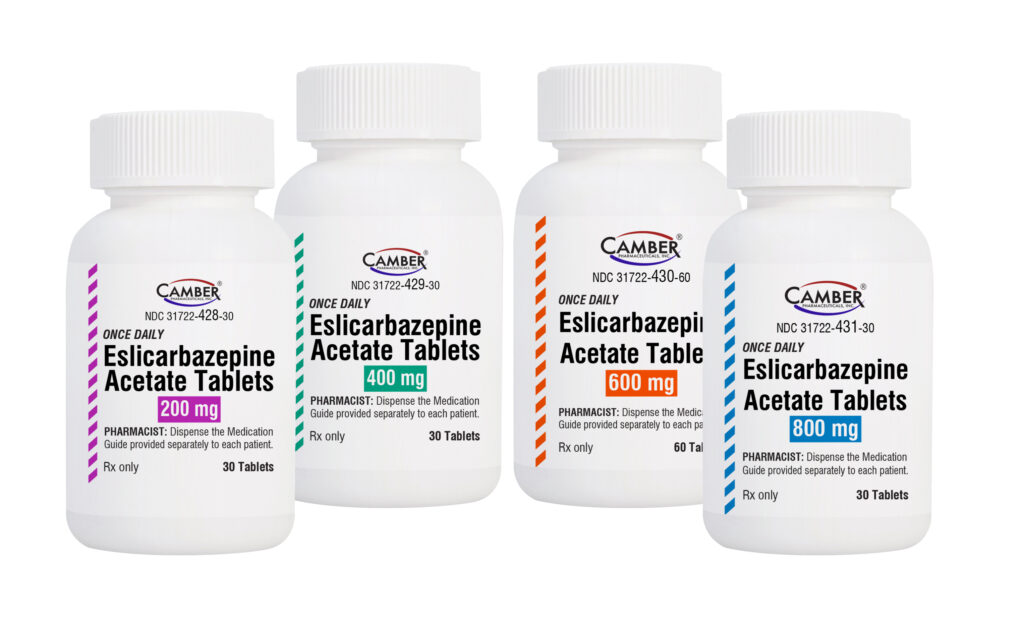
Piscataway, NJ, May 6, 2025 –Camber Pharmaceuticals is excited to announce the addition of Eslicarbazepine Acetate Tablets to its portfolio.
Eslicarbazepine Acetate Tablets are indicated for the treatment of partial-onset seizures in patients 4 years of age and older.
Eslicarbazepine Acetate Tablets are available in:
- 200 mg, 400 mg and 800 mg strengths in 30-count bottles
- 600 mg strength in a 60-count bottle
To find out more information on Eslicarbazepine Acetate Tablets, please visit: www.camberpharma.com/eslicarbazepine