Sacubitril and Valsartan Tablets
Entresto
Strengths & Sizes
Note: product and packaging images are not actual size
- NDC#31722-673-60
- UPC Code331722673600
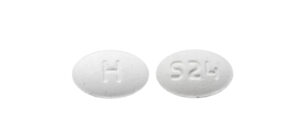
- ImprintH / S24
Note: product and packaging images are not actual size
- NDC#31722-673-18
- UPC Code331722673181
- ImprintH / S24
Note: product and packaging images are not actual size
- NDC#31722-674-60
- UPC Code331722674607
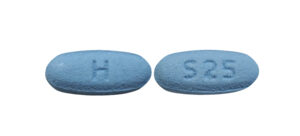
- ImprintH / S25
Note: product and packaging images are not actual size
- NDC#31722-674-18
- UPC Code331722674188
- ImprintH / S25
Note: product and packaging images are not actual size
- NDC#31722-675-60
- UPC Code331722675604
- ImprintH / S26
Note: product and packaging images are not actual size
- NDC#31722-675-18
- UPC Code331722675185
- ImprintH / S26