Camber Pharmaceuticals Launches Generic Toviaz®
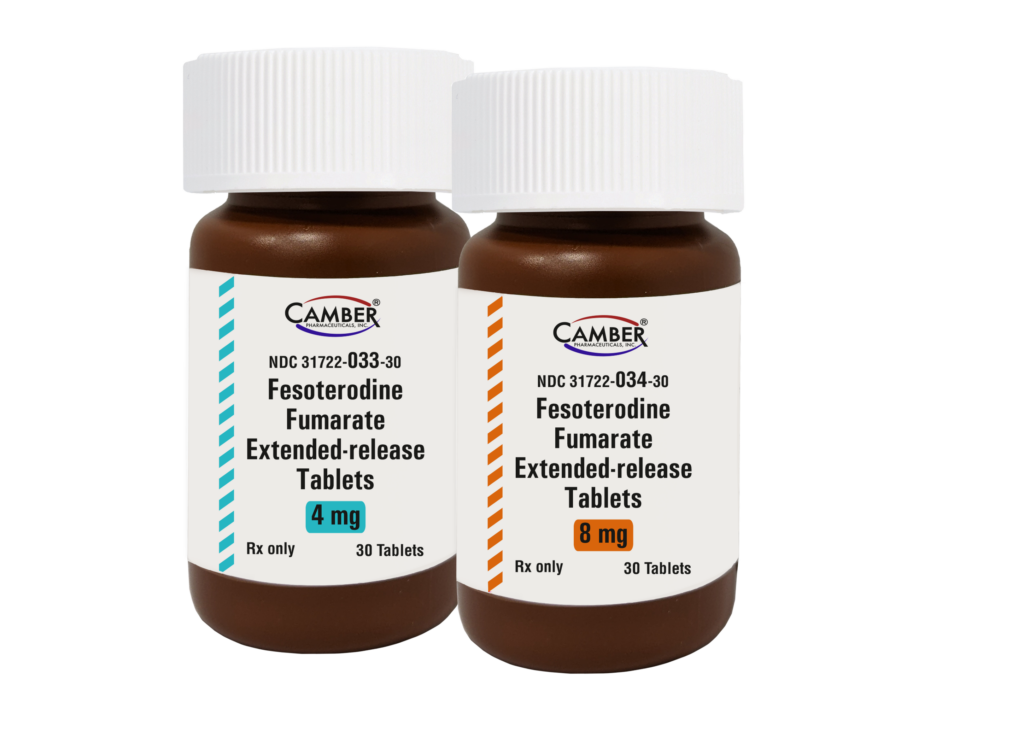
Piscataway, NJ, April 17, 2024– Camber Pharmaceuticals is excited to introduce Fesoterodine Fumarate Extended-Release Tablets.
According to the Cleveland Clinic, approximately 33 million Americans struggle with overactive bladders, impacting about 30% of adult males and 40% of adult females nationwide.
Fesoterodine Fumarate ER Tablets are indicated for the treatment of overactive bladder (OAB) in adults with symptoms of urge urinary incontinence, urgency, and frequency.
Fesoterodine Fumarate ER Tablets are available in 30 count bottles in 4 mg and 8 mg strengths.
To learn more about Fesoterodine Fumarate Extended- Release Tablets, please visit https://www.camberpharma.com/fesoterodine-fumarate-er