Camber Pharmaceuticals Launches Generic Thyro-Tabs®
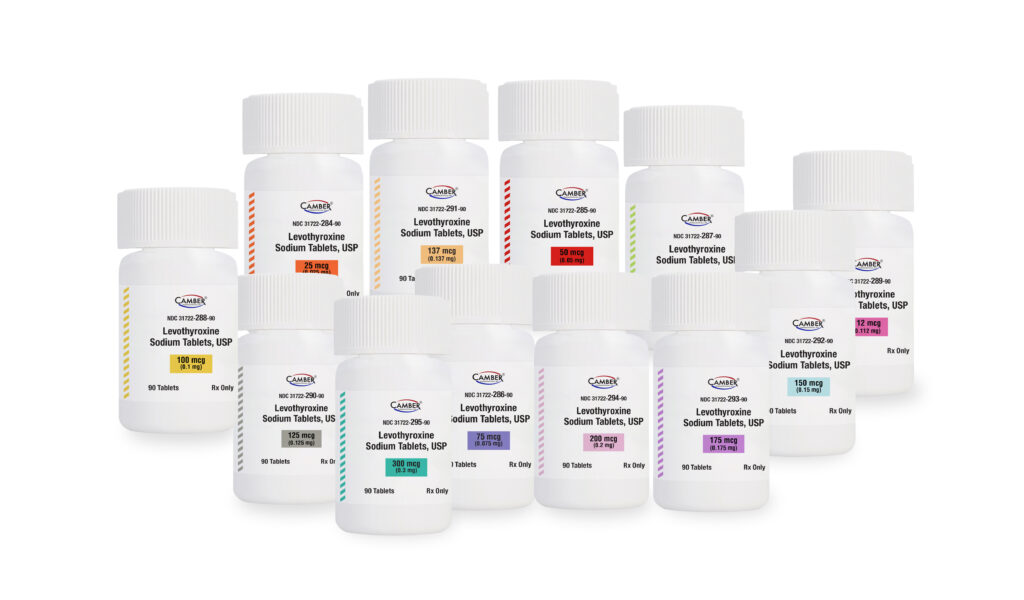
Piscataway, NJ, March 21, 2023–Camber Pharmaceuticals is pleased to announce the addition of Levothyroxine Sodium Tablets, USP to its current portfolio.
Levothyroxine Sodium Tablets, USP are indicated as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism, and as an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent well-differentiated thyroid cancer.
Levothyroxine Sodium Tablets, USP are available in 25, 50, 75, 88, 100, 112, 125, 137, 150, 175, 200, and 300 mcg strengths and supplied in 90 and 1000 count bottles.
To find out more about Levothyroxine Sodium Tablets, USP please visit www.camberpharma.com