Camber Launches Magnesium Sulfate Injection
Camber, a leader in generic drug approvals and launches, is proud to add Magnesium Sulfate Injection, USP [mag-NEE-see-um-SUL-fate] to its growing portfolio.
Magnesium Sulfate Injection, USP is suitable for replacement therapy in magnesium deficiency, especially in acute hypomagnesemia accompanied by signs of tetany similar to those observed in hypocalcemia.
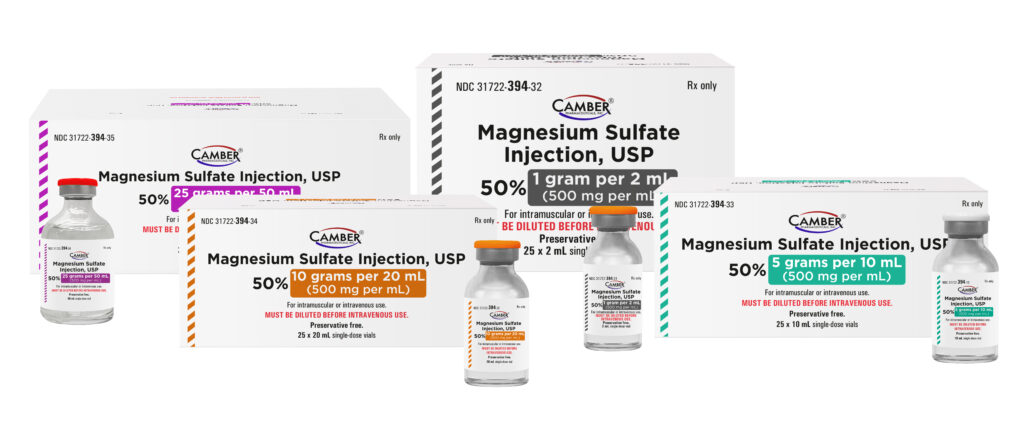
Magnesium Sulfate Injection, USP is available in:
| Strength | Size |
| 1g / 2mL (500mg per mL) * | 25 x 2 mL Single-Dose Vials |
| 5g / 10mL (500mg per mL) | 25 x 10 mL Single-Dose Vials |
| 10g / 20mL (500mg per mL) | 25 x 20 mL Single-Dose Vials |
| 25g / 50mL (500mg per mL) * | 25 x 50 mL Single-Dose Vials |
*Camber Pharmaceuticals has 180-day exclusivity on 1g / 2mL (500mg per mL) and 25g / 50mL (500mg per mL).
To learn more about this product, visit: www.camberpharma.com/magnesium-sulfate