Camber Launches Chlorpromazine HCl Injection, USP
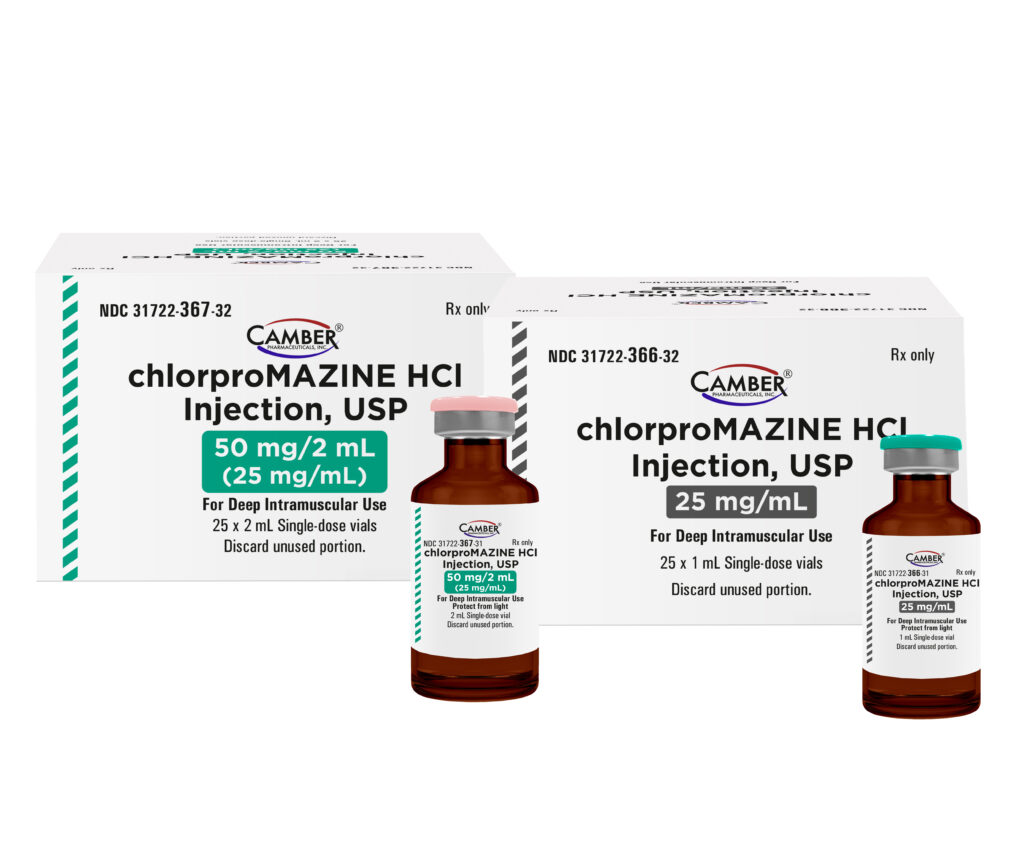
Piscataway, NJ, June 19, 2025 –Camber Pharmaceuticals is excited to announce the addition of Chlorpromazine HCl Injection, USP to its current portfolio.
Chlorpromazine HCl Injection, USP are indicated for the treatment of schizophrenia; to control nausea and vomiting; for relief of restlessness and apprehension before surgery; for acute intermittent porphyria; as an adjunct in the treatment of tetanus; to control the manifestations of the manic type of manic-depressive illness; for relief of intractable hiccups; for the treatment of severe behavioral problems in children (1 to 12 years of age) marked by combativeness and/or explosive hyperexcitable behavior (out of proportion to immediate provocations), and in the short-term treatment of hyperactive children who show excessive motor activity with accompanying conduct disorders consisting of some or all of the following symptoms: impulsivity, difficulty sustaining attention, aggressivity, mood lability, and poor frustration tolerance.
Chlorpromazine HCl Injection, USP are available in:
| Strength | Size |
|---|---|
| 25 mg/mL | 25 x 1 mL Single -dose vials |
| 50 mg/2 mL (25 mg/mL) | 25 x 2 mL Single-dose vials |
To find out more information on Chlorpromazine HCl Injection, USP, please visit: www.camberpharma.com/chlorpromazineinjection