Camber Launches Phenylephrine Hydrochloride Injection, USP
Piscataway, NJ, May 1, 2025 –Camber Pharmaceuticals is excited to announce the addition of Phenylephrine Hydrochloride Injection, USP to their portfolio.
Phenylephrine Hydrochloride Injection, USP is indicated for the treatment of clinically important hypotension resulting primarily from vasodilation in the setting of anesthesia.
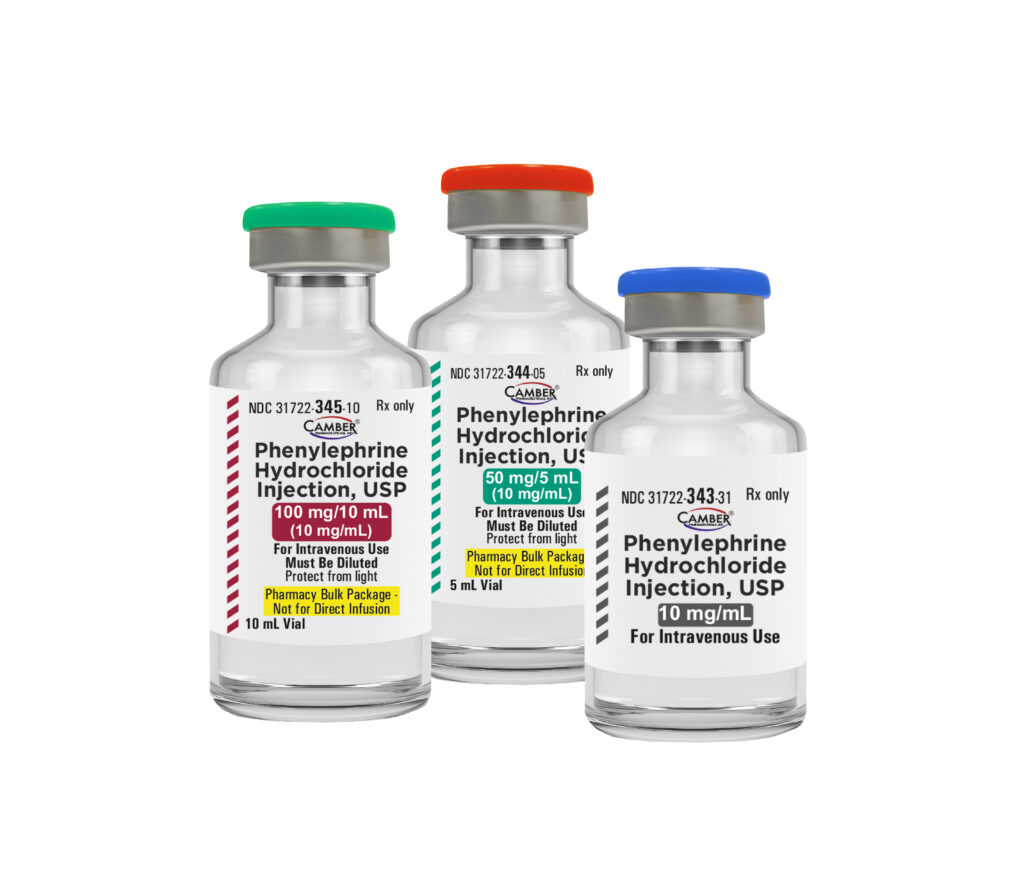
Phenylephrine Hydrochloride Injection, USP are available in:
10 mg/mL | 25 x 1 mL Single-Dose Vials
50 mg/5 mL (10 mg/mL) | 10 x 5 mL Vials
100 mg/10 mL (10 mg/mL) | 10 mL Vial
To find out more information on Phenylephrine Injection, USP , please visit: www.camberpharma.com/phenylephrine
Camber Pharmaceuticals is a fully integrated pharmaceutical company. Its parent company, Hetero, of Hyderabad, India, is one of the world’s leading API and finished dose manufacturers. Camber is committed to bringing the highest quality generic pharmaceuticals to consumers and improving quality of life through cost-effective medications.






